A high quality excipient for controlled-extended release of drugs, for industrial usage.
PLGA™
PLGA- Poly(DL-Lactide-co-Glycolide)
Applications detail
Applications
- Introduction
Mitsui PLGA is a high quality excipient for controlled-extended release of drugs, for industrial usage.
Advantages of Mitsui Chemicals' PLGA include:
- Least Residual Solvent
- Well-Controlled Molecular Weight
- Drug Master File Registered with
- FDA (USA)
- Health Canada (Canada)
- Manufactured under cGMP
PLGA Properties
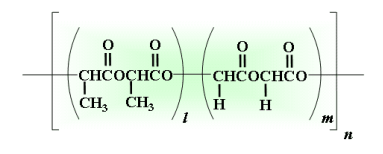
- Degraded into glycolic acid and lactic acid by hydrolysis
- Specific rate of degradation depending on its copolymer ratio and molecular weight
Physical Properies
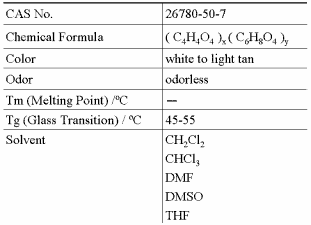
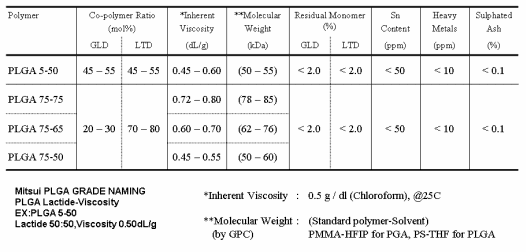
Properties of the Standard Grade
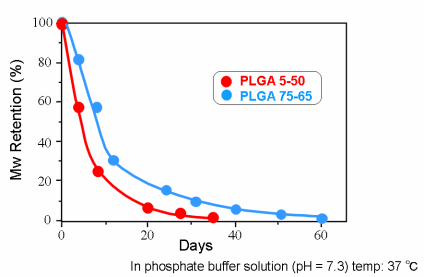
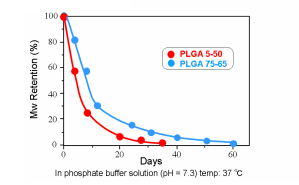
Hydrolysis of PLGA in vitro (Molecular Weight)
We welcome your requests on customize products, for more than a demand of 5kg/year.
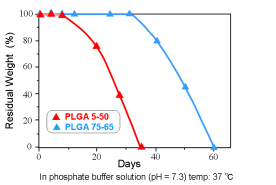
Hydrolysis of PLGA in vitro (Residual Weight)
Hydrolysis of PLGA in vitro (Molecular Weight)
This product is only for industrial (Commercial) usage.
Those in the pharmaceutical industry with a PLGA demand equal to or more than 5kg/year are encouraged to contact us.
Contact Us
MITSUI CHEMICALS AMERICA, INC.
TEL
+1 914-253-0777
FAX
+1 914-253-0790
